Week 10-11. Synaptic Connectivity
Contents
Week 10-11. Synaptic Connectivity¶
This lab requires simultaneous measurement of voltage activity in Motor Nerve 3 and a Superficial Flexor Muscle Cell of the crayfish.
Arthropod neuromuscular junctions¶
Arthropod muscle innervation is different from vertebrate muscle innervation in several interesting ways:
Arthropod muscles are innervated by relatively few excitatory motor neurons (sometimes only one).
Arthropod motor neurons innervate each muscle fiber at multiple points (multiterminal innervation).
More than one motor neuron may innervate one muscle fiber (polyneuronal innervation).
Inhibitory motor neurons may innervate muscle fibers (and sometimes the terminals of the excitatory motor nerve endings).
The tonic superficial flexor does not have “all-or-none” propagated action potentials, but instead has graded electrical responses dependent upon the level of the excitation and inhibition. The degree of depolarization determines the amount of Ca2+ that enters the cell through voltage-gated channels; the amount of Ca2+ entry in turn determines the strength of muscle contraction. Note: unlike the superficial flexor, fast phasic crayfish muscles may fire Ca2+-based action potentials.
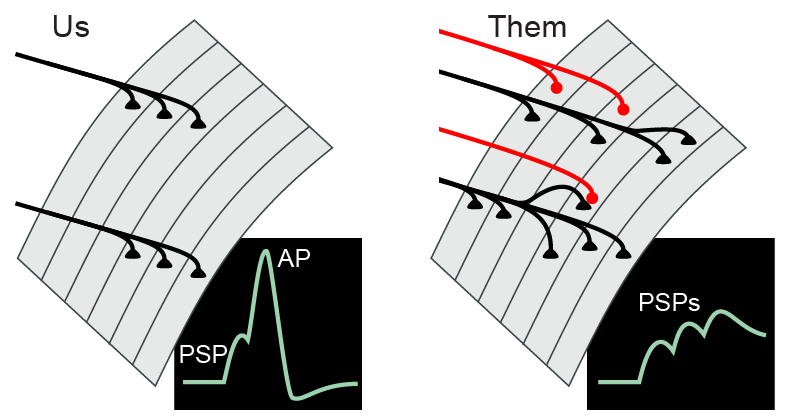
Therefore, arthropod skeletal muscle integration (innervation and summation) is actually more analagous to cortical dendritic integration in vertebrates. As in human brains, glutamate is an excitatory transmitter and GABA is an inhibitory transmitter. The multiterminal, polyneuronal, and inhibitory innervation of crustacean muscle, the use of glutamate and GABA as transmitters, and the extensive synaptic plasticity make the crayfish neuromuscular junction a good simplified model for the complex mix of synaptic interactions that occur in our own brains.

The Crayfish Superficial Flexor System¶
In crayfish, the ventral branch of the third nerve (N3v) in each abdominal ganglia innervates the superficial flexor muscle in the corresponding segment of the tail. The SF is a thin muscle sheet on the ventral surface of each tail segment, immediately under the cuticle (skin). N3v is purely motor, with no sensory axons. Thus, all of the action potentials recorded from this nerve are commands that set the contractile state of the muscle and consequently contributing to tail posture. The superficial muscle functions mostly in maintaining the posture of the animal. Motor neurons of the N3v are tonically active while the animal is at rest or performing terrestrial walking (Moore and Larimer, 1988). In contrast, motor neurons that innervate deep muscles instead generate brief bursts of action potentials associated with powerful tail-flip movements.
Experimental Goals¶
In this lab, your ultimate goals are to:
Record extracellularly the spontaneous and evoked activity in the nerve 3. Based on the amplitudes and patterns of action potentials, you will hypothesize how many axons are present in the nerve.
Record intracellularly from the superficial flexor muscle and examine spontaneous post-synaptic potentials.
Simultaneously record extracellularly from nerve 3 and intracellularly from the superficial flexor muscle, observing spontaneous postsynaptic potentials in the muscle and matching them to action potentials in the nerve. These recordings will illustrate basic principles of synaptic integration and can allow you to map the innervation of the muscle.
If the simultaneous recordings are going well, you can stimulate nerve 3 and record the elicited postsynaptic potentials in the superficial flexor muscle. This method can be used to demonstrate synaptic plasticity at the neuromuscular junction, including facilitation, synaptic depression, and long-term potentiation.
Additional Resources¶
Atwood HL (1976). Organization and synaptic physiology of crustacean neuromuscular systems. Prog Neurobiol 7:291-391. doi
Atwood HL (1982). Synapses and neurotransmitters. In: Atwood HL, Sandeman DC (eds.), The Biology of Crustacea, Vol 3, Neurobiology: Structure and Function (Academic Press, New York), ch. 3.
Atwood HL (2008). Parallel ‘phasic’ and ‘tonic’ motor systems of the crayfish abdomen. J Exp Biol 211:2193-2195. doi
Barthe J-Y, Bevengut M, Clarac F (1993). In vitro protolin and serotonin induced modulations of the abdominal motor system activities in crayfish. Brain Res 623:101-109. doi
Bishop CA, Krouse ME, Wine JJ (1991). Peptide cotransmitter potentiates calcium channel activity in crayfish skeletal muscle. J Neurosci 11:269-276. pdf
Bishop CA, Wine JJ, Nagy F, O’Shea MR (1987). Physiological consequences of a peptide cotransmitter in a crayfish nerve-muscle preparation. J Neurosci 7:1769-1779. pdf
Clement JF, Taylor AK, Velez SJ (1983). Effect of a limited target area on regeneration of specific neuromuscular connections in the crayfish. J Neurophysiol 49:216-226. pdf
Drummond JM, Macmillan DL (1998). The abdominal motor system of the crayfish, Cherax destructor. I. Morphology and physiology of the superficial extensor motor neurons. J Comp Physiol A 183:583-601. doi
Drummond JM, Macmillan DL (1998). The abdominal motor system of the crayfish, Cherax destructor. II. Morphology and physiology of the deep extensor motor neurons. J Comp Physiol A 183:603-619. doi
Evoy WH, Beranek R (1972). Pharmacological localization of excitatory and inhibitory synaptic regions in crayfish slow abdominal flexor muscle-fibres. Comp Gen Pharmacol 3:178-186. doi
Hoyle G (1983). Muscles and their Neural Control (John Wiley and Sons, New York), pp. 483-525.
Kennedy D, Evoy WH, Fields HL (1966). The unit basis of some crustacean reflexes. Symp Soc Exp Biol 20:75-109. pdf
Kennedy D, Takeda K (1965). Reflex control of abdominal flexor muscles in the crayfish II. The tonic system. J Exp Biol 43:229-246. pdf
Larimer JL (1988). The command hypothesis: A new view using an old example. Trends Neurosci 11:506-510. doi
Larimer JL, Moore D (2003). Neural basis of a simple behavior: Abdominal positioning in crayfish. Microsc Res Tech 60:346-359. doi
Leise EM, Hall WM, Mulloney B (1986). Functional organization of crayfish abdominal ganglia: I. The flexor systems. J Comp Neurol 253:25-45. doi
McCarthy BJ, Macmillan DL (1999). Control of abdominal extension in the freely moving intact crayfish Cherax destructor. I. Activity of the tonic stretch receptor. J Exp Biol 202:171-181. pdf
Murphy BF, Larimer JL (1991). The effect of various neurotransmitters and some of their agonists and antagonists on the crayfish abdominal positioning system. Comp Biochem Physiol C 100:687-698. doi
Vélez SJ, Wyman RJ (1978a). Synaptic connectivity in a crayfish neuromuscular system: 1. Gradient of innervation and synaptic strength. J Neurophysiol 41:75-84. pdf
Vélez SJ, Wyman RJ (1978b). Synaptic connectivity in a crayfish neuromuscular system: 2. Nerve-muscle matching and nerve branching patterns. J Neurophysiol 41:85-96. pdf
Wine JJ, Mittenthal JE, Kennedy D (1974). Structure of tonic flexor motoneurons in crayfish abdominal ganglia. J Comp Physiol 93:315-335. doi